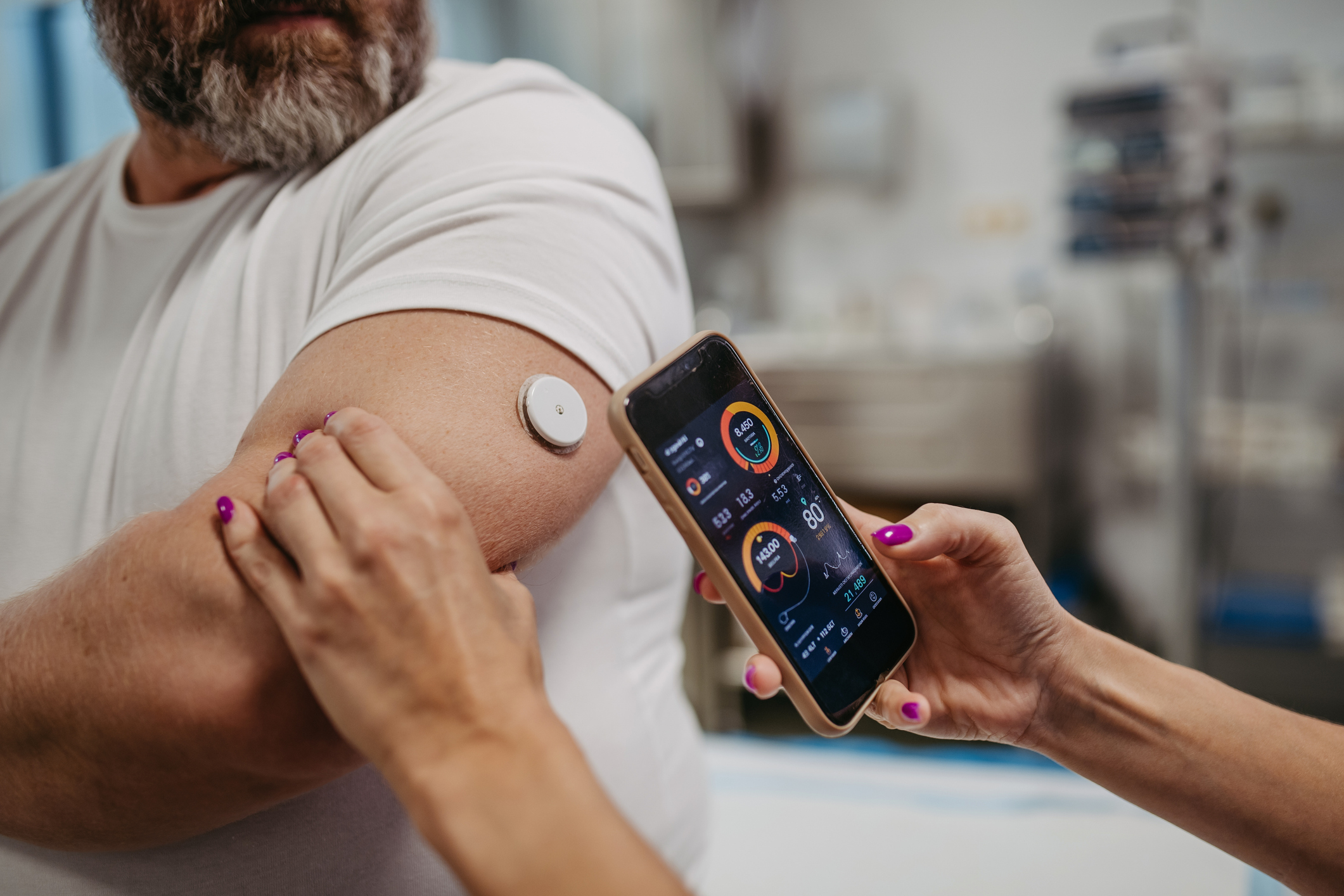
The landscape of healthcare is rapidly evolving, driven significantly by the integration of wireless technology into medical devices. From continuous glucose monitors that transmit data directly to a smartphone, to remote patient monitoring systems, and even wirelessly controlled surgical tools, these innovations promise greater patient autonomy, improved diagnostics, and more efficient care delivery. However, bringing these sophisticated devices to market requires a deep understanding and careful navigation of the U.S. Food and Drug Administration (FDA) regulations.
The FDA plays a critical role in ensuring the safety and effectiveness of all medical devices in the United States, and wireless medical devices present unique considerations. Here’s a breakdown of key aspects manufacturers must address:
Understanding the Framework
The FDA classifies medical devices into three classes (Class I, II, and III) based on their potential risks. The higher the class, the more stringent the regulatory controls. Wireless medical devices can fall into any of these classes, depending on their intended use and the risks they pose.
Key Considerations for Wireless Functionality
Wireless capabilities introduce specific challenges that the FDA meticulously scrutinizes. Manufacturers must demonstrate that the wireless features do not compromise the device’s safety or effectiveness.
- Electromagnetic Compatibility (EMC): Wireless devices emit and are susceptible to electromagnetic interference (EMI). The FDA requires robust EMC testing to ensure the device functions correctly without being adversely affected by, or adversely affecting, other electronic devices in its environment. This is crucial in clinical settings where multiple devices operate simultaneously.
- Wireless Coexistence: This goes hand-in-hand with EMC. Manufacturers must demonstrate that their device can coexist with other wireless technologies operating in the same environment without performance degradation. This involves testing scenarios where multiple wireless devices are active.
- Radiofrequency Exposure (SAR): As discussed in previous blogs, for devices that operate close to the body, manufacturers must demonstrate compliance with Specific Absorption Rate (SAR) limits to ensure safe levels of RF energy absorption by human tissue.
- Human Factors and Usability: The user interface for wireless functionalities needs to be intuitive and prevent use errors. This is particularly important for devices used directly by patients or caregivers.
- Data Reliability and Performance: For devices that transmit critical patient data, the reliability, accuracy, and timeliness of that data transfer must be validated.
Best Practices for Manufacturers
- Engage Early with the FDA: Don’t wait until your device is fully developed. Early communication through pre-submission meetings can clarify regulatory pathways and address potential challenges.
- Partner with Specialized Testing Facilities: Leverage the expertise of accredited testing laboratories. Facilities like RF Exposure Lab are purpose-built to navigate the evolving regulatory landscape, offering the precise testing and comprehensive documentation required for FDA submissions. Their specialized knowledge can significantly streamline your compliance efforts and provide the reliable data the FDA requires.
Wireless medical devices hold immense promise for transforming healthcare. However, the FDA’s rigorous regulatory framework is designed to ensure that these innovations are introduced safely and effectively. By proactively addressing EMC, wireless coexistence, robust cybersecurity, RF exposure, and usability, and by strategically partnering with experienced and adaptable testing facilities such as RF Exposure Lab, manufacturers can successfully navigate the regulatory landscape and bring life-changing wireless medical technologies to patients who need them most.
Get SAR Testing for Wireless Medical Devices at RF Exposure Lab
Our team’s expertise comes from RF Exposure Lab’s Owner, Vice President, and Chief Engineer, Jay Moulton. An authority in SAR testing with a background in manufacturing and the regulatory side of SAR testing, Jay Moulton has more than 25 years of experience. He is unique in his ability, experience, and knowledge of SAR. It’s this distinctive expertise and knowledge that allows us to guarantee our clients accurate SAR testing results and solutions.
In addition to this expertise, our team always works hard to go above and beyond, making sure that our clients understand how we are testing their devices and how SAR standards affect this. We strive to be as communicative and transparent as possible throughout the testing process so our clients are always up to date on the status of their testing.
We offer SAR testing services for a variety of wireless devices, such as
- Millimeter wave devices
- Near field charging devices
- Cell phones, laptops, and tablets
- Medical devices
- Modems
As well as many more devices! If you’re looking for SAR testing help that is provided with expertise, speed, accuracy, and integrity, contact us to learn more about our services or to get a quote.